116. Conservation of Structure and Dynamic Behavior in Triazine Macrocycles with Opportunities for Subtle Control of Hinge Motion. Patterson-Gardner, C. J.; Pan, H.; Janesko, B. G. Simanek, E. E. Org. Biomol. Chem. 2025 In press.
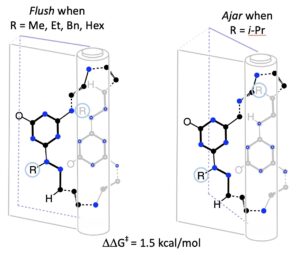
Modifying the backbone of 24-atom macrocycles allows tailoring of physical properties (octanol–water partition coefficients, log P) while conserving both conformation and the barrier to dynamic, hinge-like motion. Structure is determined by 1D and 2D NMR spectroscopy. The barrier can be subtly tuned by modifications that appear to preclude fully revolute motion and efficient π-stacking of the two subunits. The log P values increase as expected across this series, and the correlation between computed and observed values reinforces the belief of common structure and behavior across this class of macrocycles.
115. Installation of an Indole on the BRCA1 Disordered Domain Using Triazine Chemistry. Claton, L. E.; Baker, C.; Martin, H.; Dzyuba, S. V.; Zaman, K.; Prokai, L.; Stewart, M. D.; Simanek, E. E. Biomolecules 2024 In press.
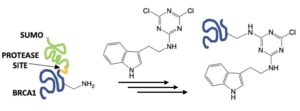
The functionalization of protein sidechains with highly water-soluble chlorotriazines (or derivatives thereof) using nucleophilic aromatic substitution reactions has been commonly employed to install various functional groups, including poly(ethylene glycol) tags or fluorogenic labels. Here, a poorly soluble dichlorotriazine with an appended indole is shown to react with a construct containing the disordered domain of BRCA1. Subsequently, this construct can undergo proteolytic cleavage to remove the SUMO-tag: the N-terminal poly(His) tag is still effective for purification. Steady-state fluorescence, circular dichroism spectroscopy, and isothermal titration calorimetry with the binding partner of BRCA1, PALB2, are used to characterize the indole-labeled BRCA1. Neither the reaction conditions nor the indole-tag appreciably alter the structure of the BRCA1. Mass spectrometry confirms that the target is modified once, although the location of modification cannot be determined by tandem mass spectrometry with collision-induced dissociation due to disadvantageous fragmentation patterns.
114. Impact of Solvent and Protonation State on Rotational Barriers in [s]-Triazines. Claton, L. E.; Simanek, E. E. J. Org. Chem. 2024, 89, 5480-5484.
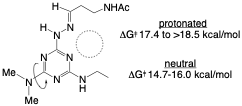
Amine-substituted [s]-triazines display hindered rotation about the triazine-N bond. For neutral triazines, this barrier has been measured to be between 15.1-17.7 kcal/mol depending on triazine substitution. However, the impact of solvent and protonation state was not addressed. Using a dimethylamine substituent as a reporter, the barrier to rotation upon protonation was measured to be 17.9 – 19.3 kcal/mol across a range of solvents (D2O, DMSO-d6, MeCN-d3, MeOD-d4, tetrahydrofuran-d8, trifluoroethanol-d3). The barrier increases as solvent dielectric decreases, a trend consistent with the role that solvent plays on stabilizing the charged heterocyclic ring. Barriers for the neutral molecule are smaller by ~ 3kcal/mol, ranging from 14.7 – 16.1 kcal/mol. The smallest barriers were recorded in polar protic solvents, an observation that could be attributed to hydrogen bonding with the reporter leading to a shift from sp2 to sp3 character of the triazine-N bond.